- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- clinical safety
Results for
Clinical safety - Import export
EC PLAZA
South Korea
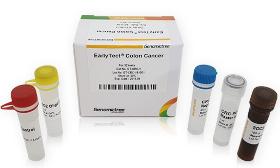
Product Description EarlyTect Colon Cancer Test was approved by the Korea Ministry of Food and Drug Safety through clinical trials at Yonsei Medical Center, Severance Hospital, and Severance Check-up Center for eligible subjects, 585 men and women aged 30 to 80. Clinical trials using fecal DNA demonstrated that CRC can be diagnosed with 90% sensitivity and specificity. EarlyTect Colon Cancer Test is a qualitative real-time PCR test that measures methylated Syndecan-2, a biomarker clinically related to precancerous lesions of CRC, in stool DNA to diagnose colorectal cancer. This product is not intended to confirm colorectal cancer, so if a subject was positive, CRC or precancerous lesions of CRC may exist. Product Standard - Cancer Type: Colorectal Cancer - Subject: Subjects for asymptomatic colorectal cancer screening - Biomarker: SDC2 me The price and transaction conditions are negotiable. Please Request a Quote for the transaction
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Clinical safety - Import exportNumber of results
1 ProductCountries
Company type
Category