- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- medical products
Results for
Medical products - Import export

PURASOL GMBH
Germany
Desinfectant, refrigerative and caring sprays, cooling sprays for sports and tattoo studios, compressed gas sprays and care products for dental and other medical equipment
Request for a quote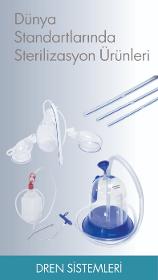
TEMAS GROUP EXPORT PARTNERS
Turkey
4A Medical Drain Drain systems are products that help collect waste blood and fluid after surgeries. The cautery pen is used for cutting and coagulating with high frequency during surgery. Our silicone and PVC drains are fully biocompatible and all test certificates are available. Our daily production capacity is around 15,000 packages, including all drains and cautery items.
Request for a quote
RECORD-TRANS
Belgium
Glassware, expensive machines or packaging for medical products... at Record-Trans we understand that the transport of delicate parcels is a highly specialised undertaking. Fortunately our team boasts both the knowledge and the means to handle your delicate items with the utmost care. Additional provisions for proper load securing Well-trained and experienced drivers Optimal safety thanks to load safety and a defensive driving style
Request for a quote
FŐNIX MMK Nonprofit és Közhasznú Kft.
Hungary
We provide specialized packaging solutions for the medical industry, ensuring that all products are safely and securely packaged to maintain their integrity
Request for a quote
HODA, UAB
Lithuania
HODA, UAB performs additional processing of plastic parts – industrial silk screen printing services. Instrument panels, molded parts, cases, control panels and bezels can be screen printed. Acrylic, aluminum, glass, stainless steel, steel and plastics can be screen printed. Screen printing can be done on curved surfaces, recessed surfaces, textured surfaces, flanges and inside boxes. Markets served include medical, consumer products, military, automotive, electronics, electrical & telecommunications.
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed

«O2 CONSULTING» LLC
Russia
The O2 Consulting experts are able to provide solutions to any legal issues that pharmaceutical companies, manufacturers, distributors, importers and developers of pharmaceuticals, medical products and equipment, private health care institutions and clinics might face. We advise our clients on licensing, registration of medical products and pharmaceuticals, transfer of technologies and other exclusive rights, organisation of clinical research and tests, import of equipment, medical products and pharmaceuticals, creation of joint ventures and distribution relations.
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
Microbiological examinations / Microbiological tests against viruses, bacteriophages, bacteria, yeast, molds, mycobacteria and bacterial spores for disinfectants, medical devices, pharmaceutical products and cosmetics Our team of experts supports manufacturers of biocidal and medical products as well as cosmetics and pharmaceuticals scientifically in the context of approval projects. For example, we create safety assessments for cosmetics and biocompatibility assessments for medical devices. Here we focus in particular on infection prevention and wound treatment products. For more information please contact INVITRO-CONNECT GmbH for assistance in developing specialized protocols for your test materials. INVITRO-CONNECT GmbH: Fast Project Execution: personal - competent - reliable --> contact@invitro-connect.com
Request for a quote
COLORICIO.SRL
Romania
The pharmaceutical salt is used for enteral and parenteral dosage forms, peritoneal dialysis solutions, penicillin production, blood plasma production, as well as an ingredient in a wide variety of medical products. In addition to its medical uses, pharmaceutical salt is also ideal for the manufacture of cosmetics, skin care and health, inhalation salt caves and for a wide range of industrial applications that require exceptional purity. Compliance with pharmacopoeia regulations is confirmed for each production batch by means of a certificate of analysis (CoA). Production is in batches and is subject to a demanding quality control process.
Request for a quote
ORBIS PRODUCTION - TOP FILM AND VIDEO PRODUCTION COMPANY IN ITALY
Italy
ORBIS Production is a Top Video Production Company in Italy and the EU, providing high-end turnkey film production services for pharmaceutical companies and institutions. Our team undergoes COVID-19 tests daily and adheres to all health safety precautions. We develop creative medical concepts, write medical scripts, shoot with the most advanced equipment, edit video, and distribute. According to the authoritative rating agency Clutch, in 2021, ORBIS Production was recognized as the top video production company in Italy. Our team professionally shoots medical videos in Italy and throughout Europe. Our trained film and video production professionals are ready for any complexity challenges, as evidenced by many successful medical video production projects. You can find AstraZeneca, University of Siena, Medical Capital, and many others among our key clients.
Request for a quote
DEDALUS LTD.
Croatia
Colors: Clear, black or white, dimension can be customized! Can be mounted vertically or horizontally Made from clear acrylic for durability and elegance Universal design for all glove brands Choose from single, double or triple to hold one, two or three boxes of gloves. +Lightweight and durable, easy to use and clean, ideal instrument for a dental office. Colors: Clear, black or white Simple structure, minimalist style, practical for dispensing dental bibs,paper towels, napkins and so on.
Request for a quote
PURASOL GMBH
Germany
Cleaner, solvent-based or without labelling obligation, technical and medical products, cosmetic products in different packagings, pump sprays, canisters, barrels
Request for a quote
PROEKT INJINIRING, LLC
Russia
A registration certificate is a document that allows you to legally convert a medical device (hereinafter referred to as a medical device, MI) on the territory of the Russian Federation. A registration certificate is issued on the basis of an Order of the registering authority (Federal Service for Supervision in the Field of Healthcare/Roszdravnadzor) after passing all the mandatory stages of state registration, during which the registration dossier is formed
Request for a quoteResults for
Medical products - Import exportNumber of results
13 ProductsCountries
Company type
Category