- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- in vitro
Results for
In vitro - Import export
DISERA MEDICAL EQUIPEMENT
Turkey
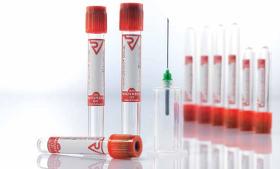
Vacusera Serum Clot Activator Tubes are used for clinical biochemistry and immunology. The inner wall of the Serum Clot Activator Tube is specially coated with clot activator, which activate coagulation process.
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
INVITRO-CONNECT GmbH offers various test strategies without animal testing for examining Eye Irritation / Eye damage hazard assessment. From standard regulatory hazard assessments to providing information on the degree of ocular irritation, to discriminating among extremely mild eye area products, INVITRO-CONNECT’ Study Monitors take the time to understand your specific goals and apply the most relevant, cost-effective strategies to achieve them. Tests for Eye Irritation / Eye damage hazard assessment in vitro: HET-CAM Assay BCOP: OECD 437 Isolated Chicken Eye Test: OECD 438 STE / Short Time Exposure (STE, OECD 491): Ocular Irritection EpiOcular & SkinEthic HCE Reconstructed human Cornea-like Epithelium (RhCE): OECD 492 For more information please contact INVITRO-CONNECT GmbH for assistance in developing specialized protocols for your test materials. INVITRO-CONNECT GmbH: Fast Project Execution: personal - competent - reliable --> contact@invitro-connect.com
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
INVITRO-CONNECT GmbH offers various test strategies without animal testing for examining Eye Irritation / Eye damage hazard assessment. From standard regulatory hazard assessments to providing information on the degree of ocular irritation, to discriminating among extremely mild eye area products, INVITRO-CONNECT’ Study Monitors take the time to understand your specific goals and apply the most relevant, cost-effective strategies to achieve them. Tests for Eye Irritation / Eye damage hazard assessment in vitro: HET-CAM Assay BCOP: OECD 437 Isolated Chicken Eye Test: OECD 438 STE / Short Time Exposure (STE, OECD 491): Ocular Irritection EpiOcular & SkinEthic HCE Reconstructed human Cornea-like Epithelium (RhCE): OECD 492 For more information please contact INVITRO-CONNECT GmbH for assistance in developing specialized protocols for your test materials. INVITRO-CONNECT GmbH: Fast Project Execution: personal - competent - reliable --> contact@invitro-connect.com
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
Determination of skin sensitization potential is a critical endpoint in the safety assessment of raw materials, chemicals, mixtures and formulations. Although the Guinea Pig Maximization Test (GPMT) and Local Lymph node assay (LLNA) have historically been used to address this adverse effect, in vitro assays have been developed and validated in order to replace these resource-intensive animal tests. INVITRO-CONNECT GmbH offers various test strategies without animal testing for examining skin sensitization: - Direct Peptide Reactivity Activation Assay (DPRA, OECD 442C) - ARE-Nrf2 Luciferase Keratinocyte Activation Test Method (OECD 442D) -Human Cell Line Activation Test (h-CLAT, OECD 442E) -Kinetic Direct Peptide Reactivity Assay (kDPRA) -Myeloid U937 Skin Sensitization Test (MUSST / U-SENS) -and more Fast Project Execution: personal - competent - reliable --> contact@invitro-connect. - More than 80 Test Laboratories - Product Safety - Regulatory Service -
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed

INVITRO-CONNECT GMBH
Switzerland
- in vitro gene mutation study in bacteria: Ames Test OECD 471 - in vitro gene mutation study in mammalian cells: Mouse Lymphoma Assay (MLTK Assay) OECD 490 - in vitro gene mutation study in mammalian cells: HPRT test / OECD 476 - Micronucleus Assay in vitro (Human Lymphocytes or V79 cells) / OECD 487 - Chromosomal aberration Assay in vitro (Human Lymphocytes or V79 cells) / OECD 473 - Hen’s Egg Test – Induction of Micronuclei (HET-MN) - COMET Assay in different cell types - Cell Transformation Assay in Bhas Cells / OECD 231 (draft) - Mammalian Erythrocyte Micronucleus Test / OECD 474 / ISO 10993-3 - Combined Mammalian Erythrocyte Micronucleus Test with concurrent Alkaline Comet Assay in vivo OECD 474 & 489 - In vivo Mammalian Alkaline Comet Assay / OECD 489 - Unscheduled DNA synthesis (UDS) Test with Mammalian Liver Cells in vivo / OECD 486 - Mammalian Spermatogonial Chromosomal Aberration test / OECD 483 - Mammalian Bone Marrow Chromosomal Aberration test / OECD 475
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
- OECD 439 / Skin Irritation Test (SIT) in a Reconstructed Human Epidermis (RhE) Model - Skin Irritation in the regulatory hazard classification and labeling context is defined as the production of reversible damage to skin following a defined chemical exposure. The Skin Irritation Test (SIT) is an in vitro, non-animal test designed to identify those chemicals and mixtures capable of inducing moderate skin irritation (UN GHS Category 2 Skin Irritants1), and to discriminate UN GHS Category 2 Skin Irritants from UN GHS 3 Mild Skin Irritants as well as those not requiring classification for skin irritation potential. INVITRO-CONNECT GmbH has extensive expertise with a wide variety of reconstructed skin-based protocols. For more information please contact INVITRO-CONNECT GmbH for assistance in developing specialized protocols for your test materials. INVITRO-CONNECT GmbH: Fast Project Execution: personal - competent - reliable --> contact@invitro-connect.com
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
- OECD 431 - Human Skin Corrosion: "In Vitro OECD 431: Reconstructed Human Epidermis Test Method, in vitro Test - Skin Corrosion in the regulatory hazard classification and labeling context is defined as the production of irreversible damage to skin, generally evident as necrosis through the epidermis and into the dermis, following a defined chemical exposure. The In Vitro Skin Corrosion Test is an in vitro, non-animal test designed to identify those chemicals and mixtures capable of inducing skin corrosion (UN GHS Category 11), and in some cases to partially subcategorize corrosives into UN GHS Sub-Categories 1A or 1B and 1C (ie., the current test methods do not effectively discriminate between UN GHS Sub-Categories 1B and 1C). For more information please contact INVITRO-CONNECT GmbH for assistance in developing specialized protocols for your test materials. INVITRO-CONNECT GmbH: Fast Project Execution: personal - competent - reliable --> contact@invitro-connect.com
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
Our experts support you with the following services: - Toxicological assessment within the framework of the REACH and biocide regulations - Environmental Risk Assessment (ERA = Environmental Impact Assessment), e.g. for pharmaceuticals - Literature research and evaluation - Examination of the reliability and validity of the studies carried out (according to OECD guidelines, EU methods or according to Klimisch et al. 1997) - Assessment of relevant toxicological endpoints, e.g. acute toxicity, genotoxicity, irritation / corrosion of the skin or eyes - Evaluation of all relevant toxicological, ecotoxicological endpoints, e.g. freshwater organisms (daphnia, algae, fish) - Read Across - Study monitoring of studies - Creation of the Endpoint Summaries and Endpoint Study Records for IUCLID (REACH and biocidal active ingredients and products) - Safety assessment and safety report for cosmetic products - Water hazard class - etc. Questions?--> contact@invitro-connect.com
Request for a quote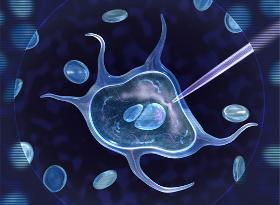
HILGENBERG GMBH
Germany
For the specialized field of in-vitro fertilization (IVF), i.e. the insemination of an egg cell outside the body, we offer various solutions. These include ready-mounted capillaries that are glued into pipettes for easier handling, as well as capillaries with Luer connection for fitting to standard disposable syringes. In-vitro fertilization is used both human medicine and in veterinary medicine. The IVF capillaries are used to hold the egg cell or to separate it from the surrounding tissue. Capillaries with different diameters are available for this purpose. Micropipettes are used to inject sperm cells into the egg cell. Capillaries for InVitro-fertilization
Request for a quote
STRATICELL
Belgium
StratiCELL offers product objectivation studies on a wide range of skin models, including cell cultures in monolayer (keratinocytes, fibroblasts, melanocytes, adipocytes, sebocytes, sensory neurons, endothelial cells, etc.), human reconstituted epidermis and finally, human skin explants. All these 2D or 3D models can be derived from primary, hiPS-derived cells or from particular conditions (i.e. from psoriatic or AD patients), and can be stimulated or exposed to stress factors as required. Our skin models are manufactured in-house and are the results of more than 15 years of R&D innovations. Skin cells in monolayer cultures In vitro reconstituted human epidermis Ex vivo skin explants
Request for a quoteResults for
In vitro - Import exportNumber of results
12 ProductsCountries
Company type