- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- implantation
Results for
Implantation - Import export

MPS MICRO PRECISION SYSTEMS AG
Switzerland
Novostia, a Swiss startup founded in 2017, has entrusted MPS with the manufacture of its TRIFLO artificial valve, a revolutionary aortic prosthesis on several levels. Thanks to its aerodynamic design, the presence of three leaflets and the absence of physical pivot axes for the leaflets, the TRIFLO valve aims to satisfy the following three criteria: - A device lifespan at least equal to that of the patient, to avoid the need for a second surgical procedure. This means more than 30 million flap openings and closures per year, with no leaks, blockages, cracks, ruptures or flap escapes. - The artificial valve must not lead to thrombosis, so that patients do not need to take anticoagulants. - Operation (flap opening and closing, blood flow through the valve) must be as silent as possible, so as not to disturb the patient or those around him, day or night. The TRIFLO device is in clinical trial and is not approved for sale.
Request for a quote
PLANSEE SE
Austria
Spare parts include for example chambers, filaments, arc slits, holders and many other parts. The pare parts are made of graphite, molybdenum, tungsten and alloys. They are extremely durable and long lasting and therefore save money and maintenance time. 100 or more Plansee components are at work in every beam path. They ensure that the ions are generated efficiently and guided precisely and free from impurities along the beam path to the wafer. For customers in the semiconductor industry, Plansee spare parts are increasingly becoming more than just spares. Taking the equipment manufacturer's original spare parts as starting point, Plansee optimizes the geometries and material compositions. The benefits to you: — Simplified component installation and removal — Longer service lives — Lower cleaning costs — Reduced maintenance work — Reduced downtimes
Request for a quote
EZM EDELSTAHLZIEHEREI MARK GMBH
Germany
EZM Edelstahlzieherei Mark is traditionally a manufacturer of speciality products, whose uses also include medical equipment. To do justice to the high, specific demands on this product segment and this market, we have given these products a special status within the company. EZM offers EZM Chirusteel and EZM Chirutan products under the brand family name EZM Chiruline. In addition to placing special orders, customers can also fall back on medical grades and versions kept in stock. EZM Edelstahlzieherei Mark offers its customers in the medical equipment sector an extensive range of materials and versions – from stainless materials to titanium, and from ground round bars to sections. Drill wires Implants/Bone plates Implants/Screws Titanium implants >> Current alloy surcharges >> Current scrap surcharges VERSIONS AND FORMS SUPPLIED Drawing From 2.0 mm to 28.0 mm round, tolerance zone IT 9 Min. bar length 2,000 mm Max. bar length 5,000 mm (2.0 mm to 6.0 mm round Max. bar...
Request for a quote
EZM EDELSTAHLZIEHEREI MARK GMBH
Germany
EZM Edelstahlzieherei Mark is traditionally a manufacturer of speciality products, whose uses also include medical equipment. To do justice to the high, specific demands on this product segment and this market, we have given these products a special status within the company. EZM offers EZM Chirusteel and EZM Chirutan products under the brand family name EZM Chiruline. In addition to placing special orders, customers can also fall back on medical grades and versions kept in stock. EZM Edelstahlzieherei Mark offers its customers in the medical equipment sector an extensive range of materials and versions – from stainless materials to titanium, and from ground round bars to sections. Drill wires Implants/Bone plates Implants/Screws Titanium implants >> Current alloy surcharges >> Current scrap surcharges VERSIONS AND FORMS SUPPLIED Drawing From 2.0 mm to 28.0 mm round, tolerance zone IT 9 Min. bar length 2,000 mm Max. bar length 5,000 mm (2.0 mm to 6.0 mm round Max. bar...
Request for a quote
PEEKCHINA CO., LTD.
China
Our wide range of biocompatible polymer materials and compounds are each designed to meet the specific and rigorous demands of implantable medical devices. PEEK materials are tailored to offer superior performance in a variety of applications including spine, orthopedic, trauma, dental and specialty devices. Innovative PEEK materials and our in-depth manufacturing knowledge combined with application expertise supports medical device companies to bring leading edge devices to market and open up new areas of growth.
Request for a quote
PEEKCHINA CO., LTD.
China
Medical-grade, biocompatible PEEK polymer materials Our wide range of biocompatible polymer materials and compounds are each designed to meet the specific and rigorous demands of implantable medical devices. PEEK materials are tailored to offer superior performance in a variety of applications including spine, orthopedic, trauma, dental and specialty devices. Innovative PEEK materials and our in-depth manufacturing knowledge combined with application expertise supports medical device companies to bring leading edge devices to market and open up new areas of growth.
Request for a quote
PEEKCHINA CO., LTD.
China
Medical-grade, biocompatible PEEK polymer materials Our wide range of biocompatible polymer materials and compounds are each designed to meet the specific and rigorous demands of implantable medical devices. PEEK materials are tailored to offer superior performance in a variety of applications including spine, orthopedic, trauma, dental and specialty devices. Innovative PEEK materials and our in-depth manufacturing knowledge combined with application expertise supports medical device companies to bring leading edge devices to market and open up new areas of growth.
Request for a quote
EZM EDELSTAHLZIEHEREI MARK GMBH
Germany
EZM Edelstahlzieherei Mark is traditionally a manufacturer of speciality products, whose uses also include medical equipment. To do justice to the high, specific demands on this product segment and this market, we have given these products a special status within the company. EZM offers EZM Chirusteel and EZM Chirutan products under the brand family name EZM Chiruline. In addition to placing special orders, customers can also fall back on medical grades and versions kept in stock. EZM Edelstahlzieherei Mark offers its customers in the medical equipment sector an extensive range of materials and versions – from stainless materials to titanium, and from ground round bars to sections. Drill wires Implants/Bone plates Implants/Screws Titanium implants >> Current alloy surcharges >> Current scrap surcharges VERSIONS AND FORMS SUPPLIED Drawing From 2.0 mm to 28.0 mm round, tolerance zone IT 9 Min. bar length 2,000 mm Max. bar length 5,000 mm (2.0 mm to 6.0 mm round Max. bar...
Request for a quote
PEEKCHINA CO., LTD.
China
Dental Disc made with PEEK polymer is a shock-absorbent, permanent solution for implant borne, fixed and removable prosthetic frameworks made with precision through CAD/CAM workflows. 1,Less inflammation Biological complications including peri-implant disease avoided in most patient cases with PEEK prostheses. 2,High implant survival Clinical studies demonstrate a high rate of implant survival in patients with a full-arch implant-supported fixed hybrid PEEK prosthethic. 3,Reduced bone loss Up to 3x-lower marginal bone loss compared to metal framework implants.
Request for a quote
PEEKCHINA CO., LTD.
China
ARK-BioPEEK offer ASTM F2026-17 compliant polyetheretherketone (PEEK) polymers designed specifically for surgical implant applications. Our advanced PEEK materials provide exceptional biocompatibility, mechanical strength, and thermal stability, meeting the stringent requirements of surgical implantation. Trust ARKPEEK and ARK-BioPEEK for high-quality PEEK solutions that ensure reliable and long-lasting performance in surgical procedures. ARK-BioPEEK introduce a range of cutting-edge polyetheretherketone (PEEK) polymers meticulously engineered for surgical implant applications. Our PEEK materials adhere to the rigorous specifications outlined in ASTM F2026-17, ensuring compliance with industry standards and delivering uncompromising quality.
Request for a quote
WESTLAKE PLASTICS EUROPE
France
Medical grade Lennite UHMWPE is produced from premium resins im accordance with ASTM specification F648 and International Standard ISO 5834-1 for surgical implants. Westlake Plastics' proprietary manufacturing processes and stringent quality control permit a well consolidated and consistent fabricated form. Recommended sterilization techniques include EtO gas, cold sterilization, and limited gamma irradiation. Often used for total hip and knee joint prosthesis, various other surgical implants and medical devices.
Request for a quote
WESTLAKE PLASTICS EUROPE
France
Westlake Plastics manufactures medical grade polymer stock shapes in rods and sheets for orthopaedic trial parts and instruments, dental applications and other surgical tools and accessories requiring medical grade standards and biocompatibility. Within its range of medical grade polymers, Westlake offers: - Polycarbonate (PC) gamma sterilized under its Zelux® GS brand name - Polyetherimid (PEI) under its Tempalux® brand name - Polypropylen (PP) under its Propylux® brand name - Polyphenylsulfon (PPSU) under the Radel® brand name - Ultra High-Molecular Weight Polethylen (UHMW-PE) under its Lennite® brand name These polymers can be used for various medical and surgical tools, knee / hip / shoulder trial parts for orthopaedic implant kits and various other medical grade applications (sterilizable cases and containers) requiring high biocompatibility specifications (USP Class VI and ISO10993).
Request for a quote
WESTLAKE PLASTICS EUROPE
France
With the new tightening procedures for cleaning and disinfecting implant ancillaries, PPSU offers an interesting solution guaranteeing a longer life cycle for products. Though it costs usually more than other medical grade polymers (POM, PP), it can nevertheless prove to be more economical in the long term due to its durability. This is especially true for complex designs which are expensive to machine. PPSU does not have any problems with the cleaning & pre-disinfection products. Our material is lot controlled, serialized and is delivered with a certificate of conformance. Coloured resin has been tested under ISO10993/5/10/11/18. The material is not for permanent implantation. PPSU behaves very well after a large number of sterilisation cycles in an autoclave at 134°C/273°F, it can be sterilized through other methods (ETO; gamma, plasma, dry heat). it is polishable for a perfect finish.
Request for a quote
MOMAN INDUSTRY
Pakistan
IMPLANT PERIODONTAL FULL INSTRUMENTS KIT 23 PCS 39-PCF1,38-TR2,43-2115,38-1516,38-ORB1/2,38-1314,38-KPA,38-PCF3,21-340B,38-BF1,38-065,33-0M9,33-BUSER,35-23CP12,25-340,31-742,15-232,15-174,12-100TC,14-202TC,14-215TC,43-130,10-020. Categories: Periodontal, Surgical
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
AMETEK SPECIALTY METAL PRODUCTS
United States
For more than 85 years, we have been at the forefront of supporting metallurgical and engineering advances in the medical industry. Today, our innovative materials are used by some of the largest manufacturers of implantable medical devices and technology in the world. Implantable Materials for Life-Saving Medical Applications Our precision strip and shaped wire materials are custom-engineered in biocompatible alloys and play a critical role in creating life-saving medical implants and medical devices. Precision Metal Strip for Implantable Medical Devices Tight Tolerance Custom Shaped Wire for Orthodontic Brackets Shaped Wire for Toothbrush Staples and Anchor Wire Electrolytic Copper Wire (C11000) used as Shaped Channel Wire for Superconductors in MRI Scanners We Engineer Precision Materials to Meet Your Medical Implant Needs Our range of highly customized medical-grade alloys combined with our metallurgical expertise, makes us the go-to material supplier
Request for a quote
AMETEK SPECIALTY METAL PRODUCTS
United States
Surgical Implant 316L is a double melted stainless steel, regarded as a medical grade, this stainless steel is melted to achieve high levels of purity and cleanliness. It has excellent resistance to both general and intergranular corrosion, and pitting and crevice corrosion. The double melting allows for superior surface finish. Surgical implant 316L Stainless Steel is our most commonly sold medical stainless steel. Beyond removing impurities, this process, in combination with the unique nickel and chromium content of 316, tends to facilitate the formation of the surface chromium oxide layer that makes stainless steel corrosion resistant. There is some belief that surgical implant 316L forms a more substantial surface layer, and that this plays a strong role in protecting the host body from reactions to the nickel content of the material. Please note that we have a minimum order value of £10,000.
Request for a quote
AMETEK SPECIALTY METAL PRODUCTS
United States
Titanium for Implantable Medical Devices Ti 6Al/4V ELI sheet has found application in medical implants owing to its excellent biocompatibility, high strength, and MRI compatibility. In the cold rolled and annealed temper the material will not exhibit a continuous alpha network at prior beta grain boundaries or a continuous alpha case layer on the surface. Owing to the alloy’s high strength and low ductility, it is difficult to cold draw into finished shapes. Hot forming has been found to be effective.
Request for a quote
AMETEK SPECIALTY METAL PRODUCTS
United States
Our titanium strip portfolio includes titanium grade 23 (Ti 6Al-4V ELI) and titanium grade 5 (Ti 6Al-4V). These products are custom-made to exacting specifications and predominantly used for implantable medical devices. We also work in partnership with Hamilton Precision Metals, part of AMETEK Specialty Metal Products, to engineer titanium strip and foil in Ti Grades 1, 2, 4 and 9 down to 1.5 microns (0.000060”) thickness. Titanium Grade 23 Strip (Ti 6Al-4V ELI) Ti 6Al/4V ELI strip is used in medical implant applications owing to its excellent biocompatibility, high strength, and MRI compatibility. Titanium Grade 5 Strip (Ti 6Al-4V) Ti 6Al/4V strip has an excellent combination of strength, corrosion resistance, and fabricability. Consequently, it is used extensively in aerospace, medical, marine, and chemical processing, and surgical implants.
Request for a quote
AMETEK SPECIALTY METAL PRODUCTS
United States
Our Pure Nickel Strip has been the premier material for battery connections for decades. The high purity of our custom-made material offers the highest conductivity of commercially manufactured nickel products due to the low impurity level. Battery Contacts for Implantable Medical Devices Patients worldwide rely on batteries to improve and maintain quality of life. Our pure nickel strip materials are used to power implantable devices such as neurostimulators, pacemakers, and cardiac defibrillators. Aerospace Battery Connectors Nickel strip engineered for high-tech aircraft battery systems that deliver long life and high reliability. Our materials are critical to safety, providing peak power for engine and emergency power backup. Rechargeable Battery Components in Drilling applications Nickel 201/270 strip for rechargeable battery components used in oilfield downhole tools, drilling, measurement
Request for a quote
AMETEK SPECIALTY METAL PRODUCTS
United States
MP35N®-LTi is a nonmagnetic nickel-cobalt-chromiummolybdenum alloy that has excellent corrosion resistance, can obtain ultrahigh tensile strengths (300 ksi) through work hardening and aging, and has good toughness and ductility. Applications include electrical components, spring contacts, medical instruments, such as stents and implant electronic connectors, chemical, food processing and marine environments. After work hardening, MP35N® alloy can be aged in the temperature range of 800/1200°F for increased strength. MP35N® will only respond to aging if in the work hardened condition. Service temperatures up to 750°F are recommended for work hardened material. MP35N® can be successfully TIG welded. MP35N® is produced by double vacuum melting, and as such, has a low nonmetallic inclusion level. Available Sizes: MP35N®-LTi is available from Hamilton Precision Metals as strip product in thicknesses from 0.0005” to 0.030” (0.0127 mm to 0.762 mm) and widths
Request for a quote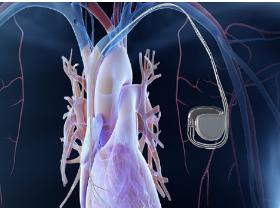
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC can provide processing of your implantable medical device components from laser cutting to the finished component.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK Engineered Medical manufactures advanced technology sensor components for different types of continuous glucose monitoring devices for its customers.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
Stent manufacturing applies to a wide variety of medical applications including cardiovascular applications, birth control, kidney stone pain control and esophageal and gastrointestinal uses.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC has been manufacturing in the orthopedic device market for decades. With regular technology improvements, we’ve adapted to the latest capabilities. Precision cutting of tube and sheet as well as precision welding with lasers is the key to our capabilities.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC offers a wide variety of laser welding capabilities for metal implant materials for the orthopedic industry.
Request for a quote
NEOSYS SURGICAL SOLUTIONS LTD.
Turkey
Arthroscopic Shoulder Instability Repair The anchor’s unique design captures suture limbs that are passed through the labrum tissue and secured within the anchor by an inner plug. Peek sizes:3.5mm, 4.5mm
Request for a quoteResults for
Implantation - Import exportNumber of results
42 ProductsCountries
Company type
Category
- Surgical implants (9)
- Import-export - medical and surgical equipment (7)
- Plastic products for the medical industry (6)
- Medical and surgical instruments (5)
- Steel & Metals (5)
- Prostheses, orthopedic (3)
- Protective and work clothing (2)
- Surgical apparatus and equipment (2)
- Disposable medical and surgical articles (1)
- Finished Metal Products (1)
- Hospitals and clinics (1)
- Medical Equipment (1)
- Pipes and tubes, stainless steel (1)
- Polyethylene (1)
- Printers - computer (1)
- Shampoos (1)
- Sheet metal and strips, tinplate (1)