- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- surgical
Results for
Surgical - Import export

MECHASYS GMBH
Germany
Medical technology: bone saws and blades for scissors If the cutting gap has to be aligned with the laser cutting of 0.07 mm, we are able to hear the smallest contours and thus filigree deeds for and a bone saw. What used to be lavishly packaged and wire-cut is now "simply" laser, and faster. Alternatively, we also cut blanks for blades, scissors, tweezers, other surgical instruments or medical instruments for endoscopy, dental and laboratory technology.
Request for a quote
OPTIMA LIFE SCIENCE GMBH
Germany
ADVANTAGES Printing: In-line flexographic printing of wallet Compliance: Complies with the industry standards of sterility Flexibility: Processes crepe and hard kraft walleting materials Validation: Fully validated, GMP, CE and OSHA compliant Optional AGPM unit, an online cuffing system that automatically handles glove placement. A link to a platen sealer for outer pouching is possible. PROCESSES Auto-Glove Placement: Automatic or manuel placement of the gloves onto the packaging web Packaging: The wallets are cut off, tucked and folded Transfer of the finished wallets to a packaging table or packaging machine - ideal: HDW Optima with headquarters in Schwaebisch Hall, Germany, teams with its subsidiaries to design and build packaging equipment for pharmaceutical, consumer, nonwoven and life science products – from single standard machines to complete complex turnkey systems.

OPTIMA LIFE SCIENCE GMBH
Germany
Advantages: Quality: high seal integrity, non-compliant packs are rejected automatically Flexibility: able to run a wide range of papers and films Validation: Fully validatable, GMP, CE and OSHA compliant Processes: Infeed Manual or automatic feeding - ideal GPM The infeed transfers the product in the correct position between two webs of material Sealing Continuous platen sealing Cutting Cut to the required length Optional inspection Integrated control cabinet Automatic reject of non-compliant packs Optima teams with its subsidiaries to design and build packaging equipment for pharmaceutical, consumer, nonwoven and life science products – from single standard machines to complete complex turnkey systems. Whether custom solutions or modular standard units, functions are consistently tailored to the specific needs of the customers and their industries. Optima is the worldwide leader in packaging technologies for many fields.

MAXCERT LTD
Germany
Maxcert is offering EC Representative services for non EU medical devices manufacturers. Maxcert is authorized/ registered EC REP service provider and can manage your ECREP requirements better than others. We make sure that you are MDR ready! and well updated with the EU regulations for your medical devices. Maxcert’s team has expert knowledge to help you navigate through the complex regulatory challenges that the new EU MDR bring. We can assist you through out the entire process to ensure that you and your business are compliant with all of the EU MDR requirements. For more information, reach out to us at
Request for a quote
MAXCERT LTD
Germany
The new European Commission’s guidance on persons responsible for regulatory compliance (PRRC) explains that manufacturers, Authorized Representatives and micro and small manufacturers must designate at least one staff member responsible for ensuring compliance to the MDR and/or IVDR as appropriate according to Article 15 of the Regulation. PRRC qualifications are specific for each of these three operators. This does not mean that you have to hire PRRC as employee rather you can avail our PRRC service to overcome the regualtory needs of your company and medical devices – with a consultant managing the system on a few days per month basis. We would be more than happy to discuss this option with you, helping you to find a consultant with the necessary qualifications to perform this service effectively. If you would like to know more about this service, then please contact us.
Request for a quote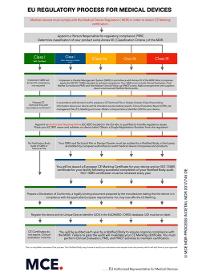
MAXCERT LTD
Germany
Our experienced team has for years done documentation for companies who are manufacturers of medical devices /surgical instruments. We provide Quality-Management-Systems for meeting legal and normative requirements. For manufacturers of medical devices / surgical instruments class I (including Is, Im, and new for MDR class Ir), IIa, and IIb we offer the following services: Implementation DIN EN ISO 13485 and the EU-Regulation 2017/745 MDR and 21 CFR 820 QSR Creating QM-Systems with procedural instructions and work instructions Technical Documentation Clinical evaluation Risk Management / Risk Analysis Essential Safety and Performance Requirements Validation of processes UDI-labeling Consulting for Regulatory Affairs Internal audits and external supplier audits Accompaniment and support with inspections by authorities and Audits by notified bodies Communication with authorities
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Surgical - Import exportNumber of results
6 ProductsCompany type