- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- exporters and importers
Results for
Exporters and importers - Import export

YSTRAL GMBH MASCHINENBAU + PROCESSTECHNIK
Germany
Paints and coatings, sealing and insulating materials, coatings, chocolate masses, dairy products, personal care and many other products are produced on process systems from our company. The efficiency and effectiveness of your manufacturing processes can be significantly increased with the help of individually built process plants. Customized process plants are used by our customers worldwide. Container systems, which can be used flexibly at different locations, are available as well as our skidded units, which are completely frame-mounted and automated production units. Recipe control, visualization, weighing, measurement and control technology, containers and piping, CIP systems - all plants are completely assembled in our factory. After a test run under production conditions they are accepted by you. Our technicians are available to you on site for installation, commissioning and service.
Request for a quote
YSTRAL GMBH MASCHINENBAU + PROCESSTECHNIK
Germany
The combination of different process steps in one machine provides you with great rationalisation potential: Production times are minimised, sub-processes are completely eliminated, production costs are reduced.The YSTRAL Conti-TDS is based on the same principle as the YSTRAL Inline Disperser. A special rotor-stator system generates a large negative pressure and absorbs powder, without dust formation and loss-free, directly into the liquid. The inducted powder is dispersed immediately and under high shear. Clean manufacturing process thanks to a dust and loss-free powder feed, even with powders that are difficult to wet, sticky or dusty Aspirating and dispersing of powders directly from silo (via buffer hopper), big bag, hopper, sack, barrel etc. Optimized result a better digestion, a higher effectiveness of the raw materials and a higher product quality.
Request for a quote
MAXCERT LTD
Germany
The new European Commission’s guidance on persons responsible for regulatory compliance (PRRC) explains that manufacturers, Authorized Representatives and micro and small manufacturers must designate at least one staff member responsible for ensuring compliance to the MDR and/or IVDR as appropriate according to Article 15 of the Regulation. PRRC qualifications are specific for each of these three operators. This does not mean that you have to hire PRRC as employee rather you can avail our PRRC service to overcome the regualtory needs of your company and medical devices – with a consultant managing the system on a few days per month basis. We would be more than happy to discuss this option with you, helping you to find a consultant with the necessary qualifications to perform this service effectively. If you would like to know more about this service, then please contact us.
Request for a quote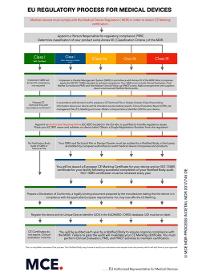
MAXCERT LTD
Germany
Our experienced team has for years done documentation for companies who are manufacturers of medical devices /surgical instruments. We provide Quality-Management-Systems for meeting legal and normative requirements. For manufacturers of medical devices / surgical instruments class I (including Is, Im, and new for MDR class Ir), IIa, and IIb we offer the following services: Implementation DIN EN ISO 13485 and the EU-Regulation 2017/745 MDR and 21 CFR 820 QSR Creating QM-Systems with procedural instructions and work instructions Technical Documentation Clinical evaluation Risk Management / Risk Analysis Essential Safety and Performance Requirements Validation of processes UDI-labeling Consulting for Regulatory Affairs Internal audits and external supplier audits Accompaniment and support with inspections by authorities and Audits by notified bodies Communication with authorities
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Exporters and importers - Import exportNumber of results
4 ProductsCompany type