- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- document management
Results for
Document management - Import export
CIC - KLAUS CZERWONKA
Germany
Taking on tasks in the field of design and development such as: CAD construction on CATIA and Pro E Component tuning installation space investigation Creation of 2-D drawings Documentation Change management
Request for a quote
FILLTECH GMBH
Germany
Top security for your processes Quality is no accident. Benefit from the highest process reliability, from the idea to the development to the finished product. More than 14 years of experience, intensive know-how in the field of gas filling and qualified employees are the basis for successful applications. As a team we work to inspire you - with competence, reliability and quality. Quality management with latest technology A continuous quality management documents all processes: Your orders are managed by the ERP-system, Microsoft AX, all quality-related data will be stored in Share Point libraries for secure storage, evaluation and documentation. Almost the entire quality management is IT based. Interfaces to proprietary software developments allow external connections to quality-related data - the highest transparency for you and your processes. Documented Reliable batch traceability: consistently, from the source to the bottle. Our currently unique cylinder database saves all impor
Request for a quote
VIALUTIONS GMBH
Germany
The Microsoft 365 world with SharePoint and Microsoft Teams facilitates collaboration enormously for various reasons. For example, dynamic team websites allow project groups to collaborate more closely, even with external teammates. Keeping people informed and involved is also made easier. There are various document management options to make information and work materials available and thus improve operational efficiency.
Request for a quote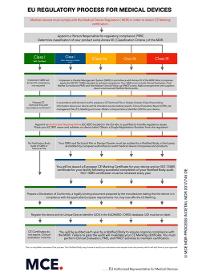
MAXCERT LTD
Germany
Our experienced team has for years done documentation for companies who are manufacturers of medical devices /surgical instruments. We provide Quality-Management-Systems for meeting legal and normative requirements. For manufacturers of medical devices / surgical instruments class I (including Is, Im, and new for MDR class Ir), IIa, and IIb we offer the following services: Implementation DIN EN ISO 13485 and the EU-Regulation 2017/745 MDR and 21 CFR 820 QSR Creating QM-Systems with procedural instructions and work instructions Technical Documentation Clinical evaluation Risk Management / Risk Analysis Essential Safety and Performance Requirements Validation of processes UDI-labeling Consulting for Regulatory Affairs Internal audits and external supplier audits Accompaniment and support with inspections by authorities and Audits by notified bodies Communication with authorities
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Document management - Import exportNumber of results
4 ProductsCountries
Company type