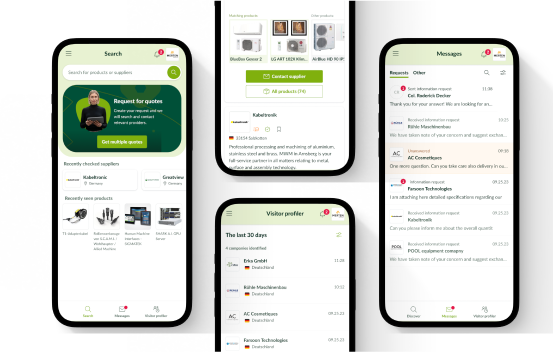
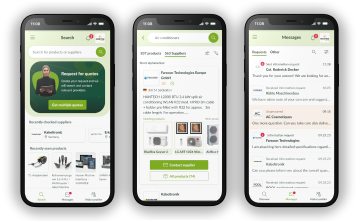
All categories
AddArmature technologyBattery and battery technologyBuilderBuilding materials and construction suppliesCable TechnologyCatering SuppliesCleaningCleaning and care productsCleaning devices and machinesCleaning utensilsClothing and AccessoriesComponentsConstruction machinery and equipmentConstruction site suppliesConsultationContainers, logistics, and warehouse suppliesConveying technologyDesignDie-casting and injection molding machinesDie casting, injection molding, and additive manufacturingDisposal and RecyclingDrive technologyElectrical EngineeringElectrical servicesEnergy TechnologyEnvironmental technologyEvent and event servicesFastening technology, connection technology, and fittingsFilter and Sieve TechnologyFinance and InsuranceFluid technologyFood TechnologyFurniture and Interior DesignGastronomyGastronomy and kitchen equipmentGlass and ceramic productsGroceriesHardware for Information and Communication TechnologyHeat TreatmentHome TextilesHousehold itemsIndustrial SuppliesInformation technology servicesLabeling technologyLaboratory equipment and suppliesLaser technologyLeather, textile fibers, yarns, and fabricsLeisure, Culture and TourismLightingLogistics, Transportation, and StorageMachine partsMachinery for agriculture, forestry, fisheries, horticultureMachine tools and equipmentMachining and formingManagement and AdministrationMarketing, Advertising, and SalesMeasurement and Testing EquipmentMeasurement, testing, and analysis servicesMechanical engineering, plant construction, and apparatus engineeringMedicine and HealthMetal and Metal ProductsMontageMultimediaNeed for agriculture, forestry, fishing, horticultureOccupational health and safetyOffice suppliesOpticsPackaging machinesPackaging materialPackaging servicesPersonal care and cosmetic productsPersonnel servicesPhoto, film, and sound servicesPipe and Hose TechnologyPlanning, Construction, and DevelopmentPlastic and plastic productsPrinting and paperPrinting and paper machinesProcess EngineeringProducts from agriculture, forestry, fisheries, horticulturePromotional materials and advertising technologyPump TechnologyPurchase and closeout dealsRaw materials and ChemistryReal estate servicesRefrigeration, air conditioning, ventilation, and heating technologyRepair, Maintenance, and ModernizationRobotics, Process, and Automation TechnologySafety and rescue technologySanitary engineeringSealing technologySecurity servicesServices from agriculture, forestry, fishing, horticultureSoftwareSoldering, Welding, and Bonding TechnologySportsSurface treatmentSurface treatment machinesTax and control technologyTextile machinesTextile productionTextile production and leather processing requirementsTools and Manufacturing SuppliesTraffic engineeringTraining and further educationTranslation and editing servicesVehicle parts and vehicle accessoriesVehiclesWood and wood products