- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- legal system
Results for
Legal system - Import export

IFA TECHNOLOGY GMBH
Germany
As a specialist company according to the German Water Resources Act (WHG), IFA Technology offers expert planning and consulting for the storage of liquids or bulk materials. In the area of so-called LAU plants and systems (plants and systems for storage, filling and handling), our product range includes tank farms and silo systems which, depending on the requirements, consist of mild steel, stainless steel, plastic and GRP tanks. To meet the legal requirements, LAU plants and systems from IFA Technology can be supplied specially coated (HALAR, Teflon, etc.), varnished or painted.
Request for a quote
SIEMPELKAMP MASCHINENFABRIK GMBH
Germany
Siempelkamp has developed the energy-ecient drive system EcoDrive for the ContiRoll® main drives. This system meets tomorrow’s legal and standard demands on the energy eciency of drive systems already today. It comprises frequency converters, motor and gearbox - all well-tuned to one another. In addition to the energy eciency our R&D-work focussed on an improved precision and controllability of the entire drive system. The focus is on high belt speeds, precise r.p.m. for a synchronous operation of the steel belts, torques adjusted to the characteristic load line in connection with the hydraulic pressure/position control, good performance, good uptimes and ease of maintenance. This corresponds to the demand on a very high product quality and an on-demand energy consumption even in partial load operation. This new drive system is able to save more than 10 % of the electrical drive energy compared to traditional Solutions.
Request for a quote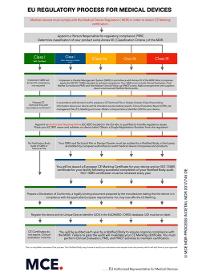
MAXCERT LTD
Germany
Our experienced team has for years done documentation for companies who are manufacturers of medical devices /surgical instruments. We provide Quality-Management-Systems for meeting legal and normative requirements. For manufacturers of medical devices / surgical instruments class I (including Is, Im, and new for MDR class Ir), IIa, and IIb we offer the following services: Implementation DIN EN ISO 13485 and the EU-Regulation 2017/745 MDR and 21 CFR 820 QSR Creating QM-Systems with procedural instructions and work instructions Technical Documentation Clinical evaluation Risk Management / Risk Analysis Essential Safety and Performance Requirements Validation of processes UDI-labeling Consulting for Regulatory Affairs Internal audits and external supplier audits Accompaniment and support with inspections by authorities and Audits by notified bodies Communication with authorities
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Legal system - Import exportNumber of results
3 ProductsCountries
Company type